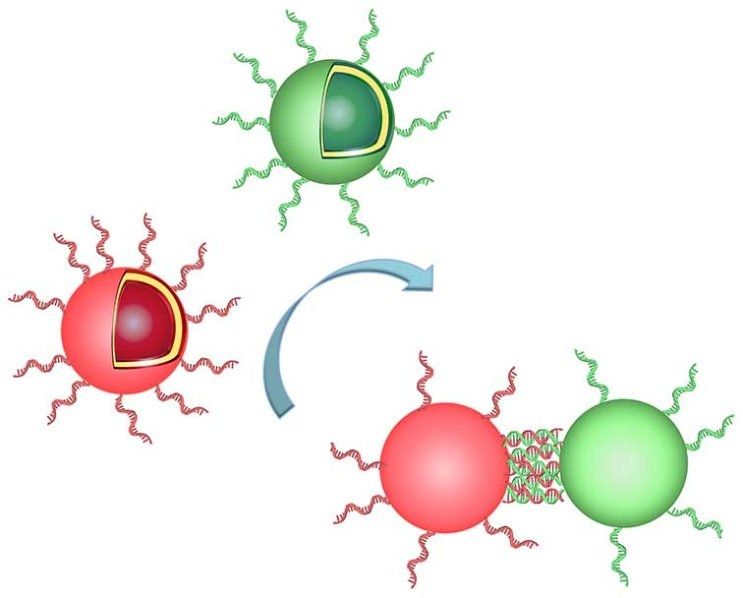
Scientists from the University of Basel have succeeded in organizing spherical compartments into clusters mimicking the way natural organelles would create complex structures. They managed to connect the synthetic compartments by creating bridges made of DNA between them. This represents an important step towards the realization of so-called molecular factories. The journal Nano Letters has published their results.
Within a cell there are specialized compartments called organelles, as for example nucleus, mitochondria, peroxisomes and vacuoles that are responsible for specific functions of the cell. Almost all sophisticated biological functions of cells are realized by self-organization, a process by which molecules adopt a defined arrangement based on their specific conformations and properties, without outside guidance.
Using self-organization of nano-objects into complex architectures is a major strategy to produce new materials with improved properties or functionalities in fields such as chemistry, electronics and technology. For example, this strategy has already been applied to create networks of inorganic solid nanoparticles. However, so far, these networks were not able to mimic sophisticated structures that have biological functions within the cells and thus have potential application in medicine or biology.
Read more