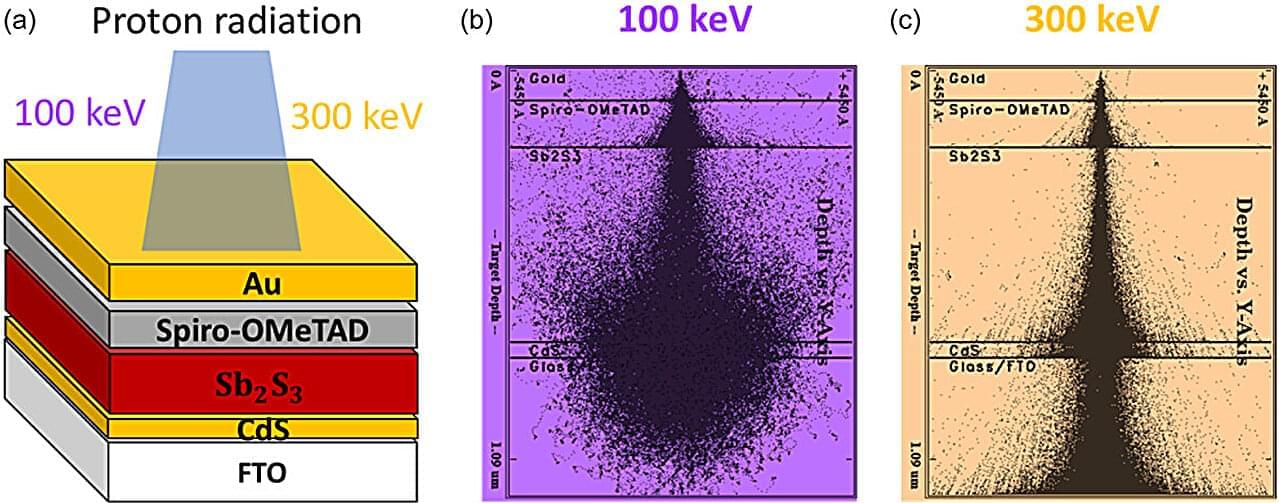
Solar cells face significant challenges when deployed in outer space, where extremes in the environment decrease the efficiency and longevity they enjoy back on Earth. University of Toledo physicists are taking on these challenges at the Wright Center for Photovoltaics Innovation and Commercialization, in line with a large-scale research project supported by the Air Force Research Laboratory.
One recent advancement pertains to an emerging technology that utilizes antimony compounds as light-absorbing semiconductors. A group of UToledo faculty and students recently published a first-of-its-kind assessment exploring the promising characteristics of these antimony chalcogenide-based solar cells for space applications in the journal Solar RRL, which highlighted the work on its front cover.
“Antimony chalcogenide solar cells exhibit superior radiation robustness compared to the conventional technologies we’re deploying in space,” said Alisha Adhikari, a doctoral student in physics who co-led the team of undergraduate, graduate and faculty researchers at UToledo. “But they’ll need to become much more efficient before they become a competitive alternative for future space missions.”