Forget the stereotype. Vegetarianism is becoming a cultural statement.


This wireless desk can power almost your entire PC, no cables or batteries needed.

The extraction of work (i.e., usable energy) from quantum processes is a key focus of quantum thermodynamics research, which explores the application of thermodynamics laws to quantum systems. Meanwhile, other quantum physics research has been investigating the non-Markovian dynamics of open quantum systems, which entail the influence of past states on the systems’ future evolution.
Researchers at the University of Nottingham and University of São Paulo have introduced a general and rigorous framework that bridges quantum thermodynamics and non-Markovian dynamics, showing that the latter could serve as a resource that can be exploited to enhance the extraction of work from quantum processes.
Their paper, published in Physical Review Letters, could open new possibilities for the future development of quantum technologies.

An international research collaboration featuring scientists from the FAMU-FSU College of Engineering and the National High Magnetic Field Laboratory has discovered a fundamental universal principle that governs how microscopic whirlpools interact, collide and transform within quantum fluids, which also has implications for understanding fluids that behave according to classical physics.
The study, which was published in the Proceedings of the National Academy of Sciences, revealed new insights into vortex dynamics within superfluid helium, a remarkable liquid that exhibits zero-resistance flow at temperatures approaching absolute zero. The research demonstrates that when these quantum vortices intersect and reconnect, they separate faster than their initial approach velocity, creating bursts of energy that characterize turbulence in both quantum and classical fluids.
“Superfluids offer a uniquely clear perspective on turbulence,” said FAMU-FSU College of Engineering Professor Wei Guo, a study co-author. “We’re beginning to understand the universal physics that connects quantum and classical worlds, and that’s an exciting frontier for both science and technology.”
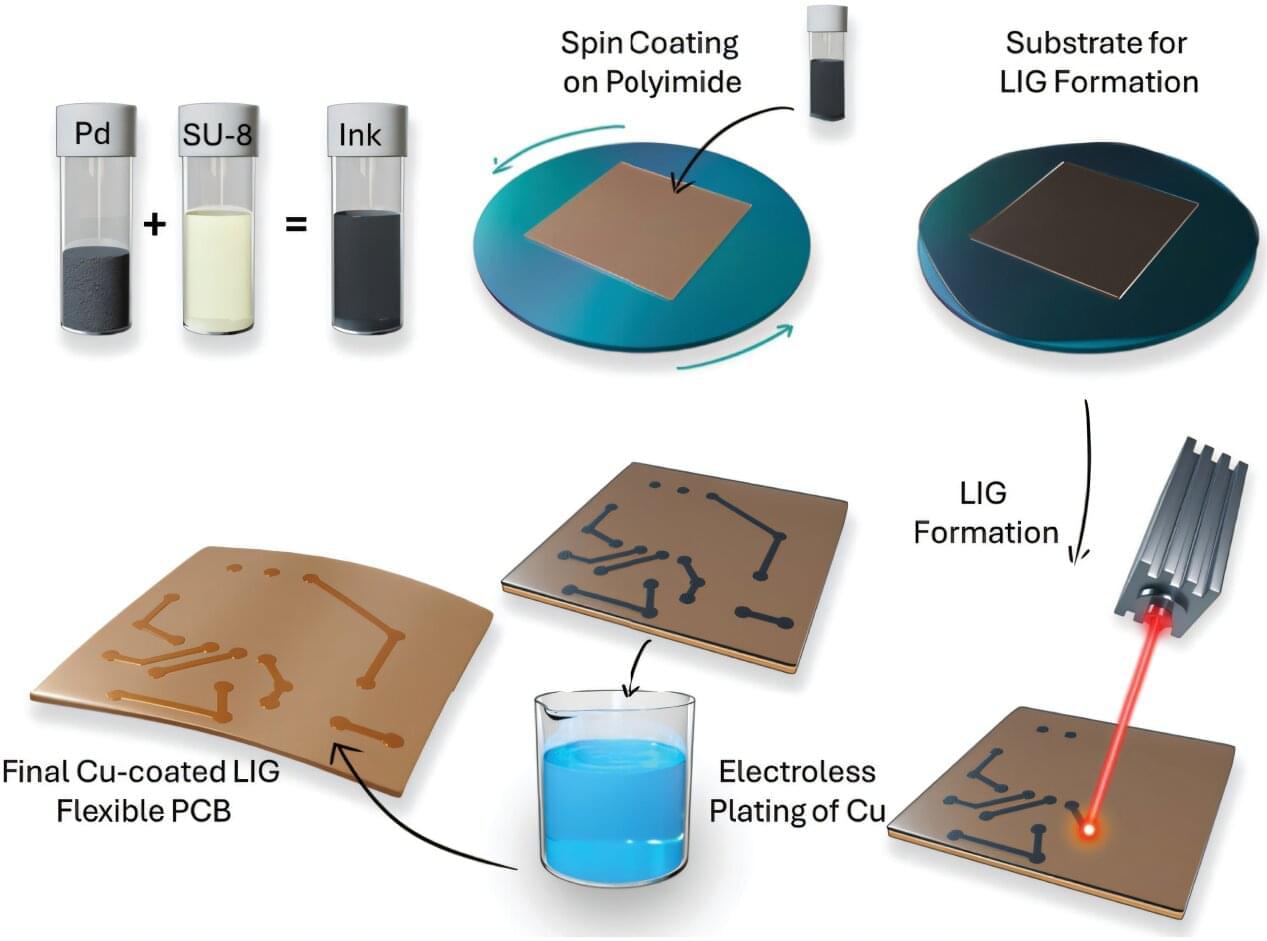
Boise State University researchers have unveiled a cutting-edge approach to manufacturing flexible hybrid circuits—reducing costs, waste, and environmental impact. Their work leverages the properties of laser-induced graphene and was recently featured on the cover of Advanced Materials Technologies.
Laser-induced graphene uses a single-step laser manufacturing process that converts carbon-rich materials into a 3-dimensional conductive and porous structure with some regions of atomically thin graphene. This technique is scalable, cost-effective, and patternable, making it ideal for applications in electronics, sensing, and energy storage.
In this work, the researchers used palladium (Pd) nanoparticles embedded in a polymer matrix to form Pd functionalized laser-induced graphene. These Pd nanoparticles act as seed crystals for the electroless deposition of copper on the LIG scaffold, thus forming copper interconnects for flexible printed circuit boards (f-PCBs) through a laser-enabled additive manufacturing process.

Turning crude oil into everyday fuels like gasoline, diesel, and heating oil demands a huge amount of energy. In fact, this process is responsible for about 6 percent of the world’s carbon dioxide emissions. Most of that energy is spent heating the oil to separate its components based on their boiling points.
Now, in an exciting breakthrough, engineers at MIT have created a new kind of membrane that could change the game. Instead of using heat, this innovative membrane separates crude oil by filtering its components based on their molecular size.
“This is a whole new way of envisioning a separation process. Instead of boiling mixtures to purify them, why not separate components based on shape and size? The key innovation is that the filters we developed can separate very small molecules at an atomistic length scale,” says Zachary P. Smith, an associate professor of chemical engineering at MIT and the senior author of the new study.
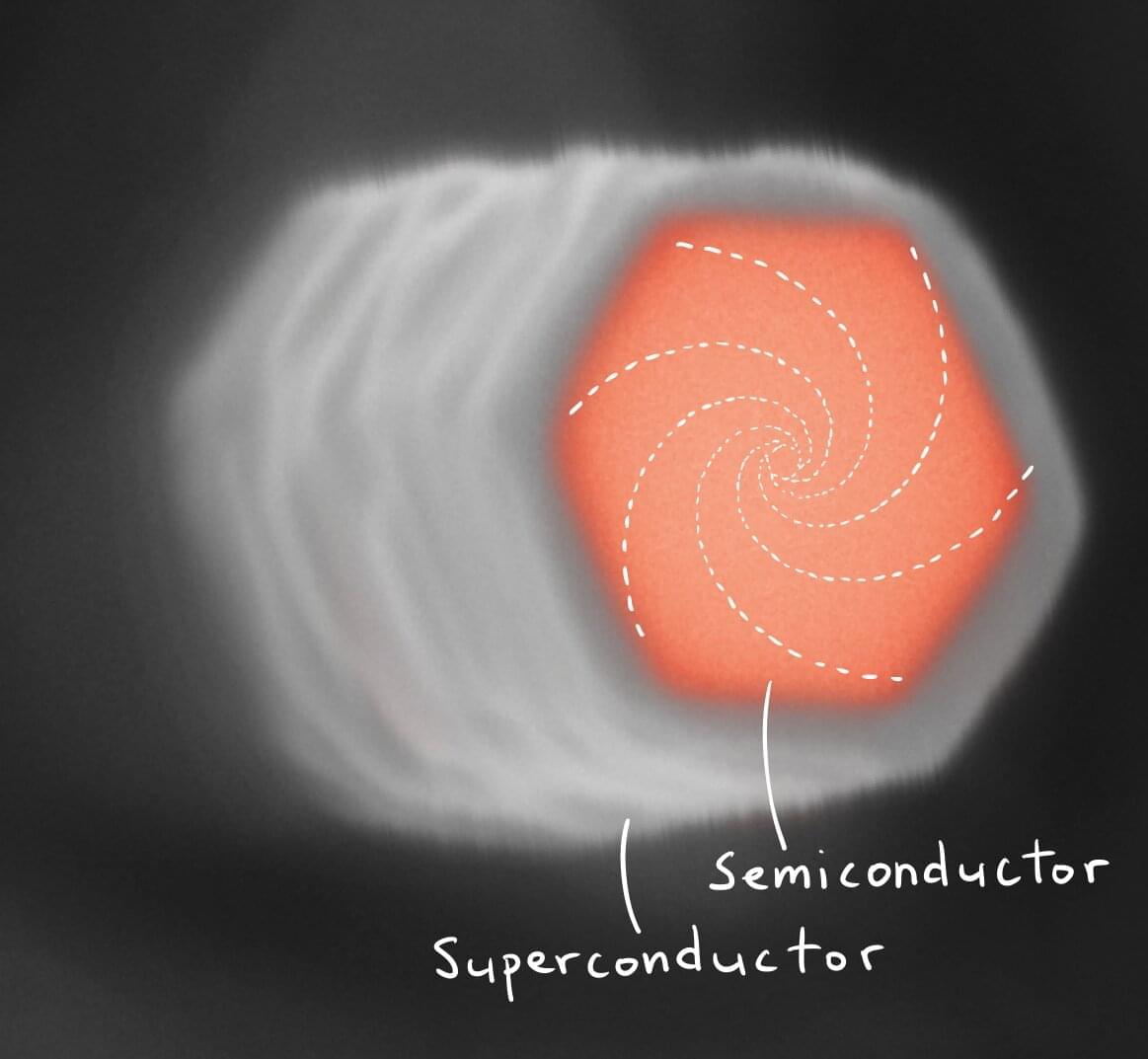
Researchers from the Niels Bohr Institute, University of Copenhagen, have created a novel pathway into the study of the elusive quantum states in superconducting vortices. The existence of these was flaunted in the 1960s, but has remained very difficult to verify directly because those states are squeezed into energy scales smaller than one can typically resolve in experiments.
The result was made possible by a combination of ingenuity and the expanding research in designer materials created in the labs at the Niels Bohr Institute. It is now published in Physical Review Letters.

Alena Tensor is a recently discovered class of energy-momentum tensors that proposes a general equivalence of the curved path and geodesic for analyzed spacetimes which allows the analysis of physical systems in curvilinear, classical and quantum descriptions. In this paper it is shown that Alena Tensor is related to the Killing tensor K and describes the class of GR solutions G + Λ g = 2 Λ K. In this picture, it is not matter that imposes curvature, but rather the geometric symmetries, encoded in the Killing tensor, determine the way spacetime curves and how matter can be distributed in it. It was also shown, that Alena Tensor gives decomposition of energy-momentum tensor of the electromagnetic field using two null-vectors and in natural way forces the Higgs field to appear, indicating the reason for the symmetry breaking.
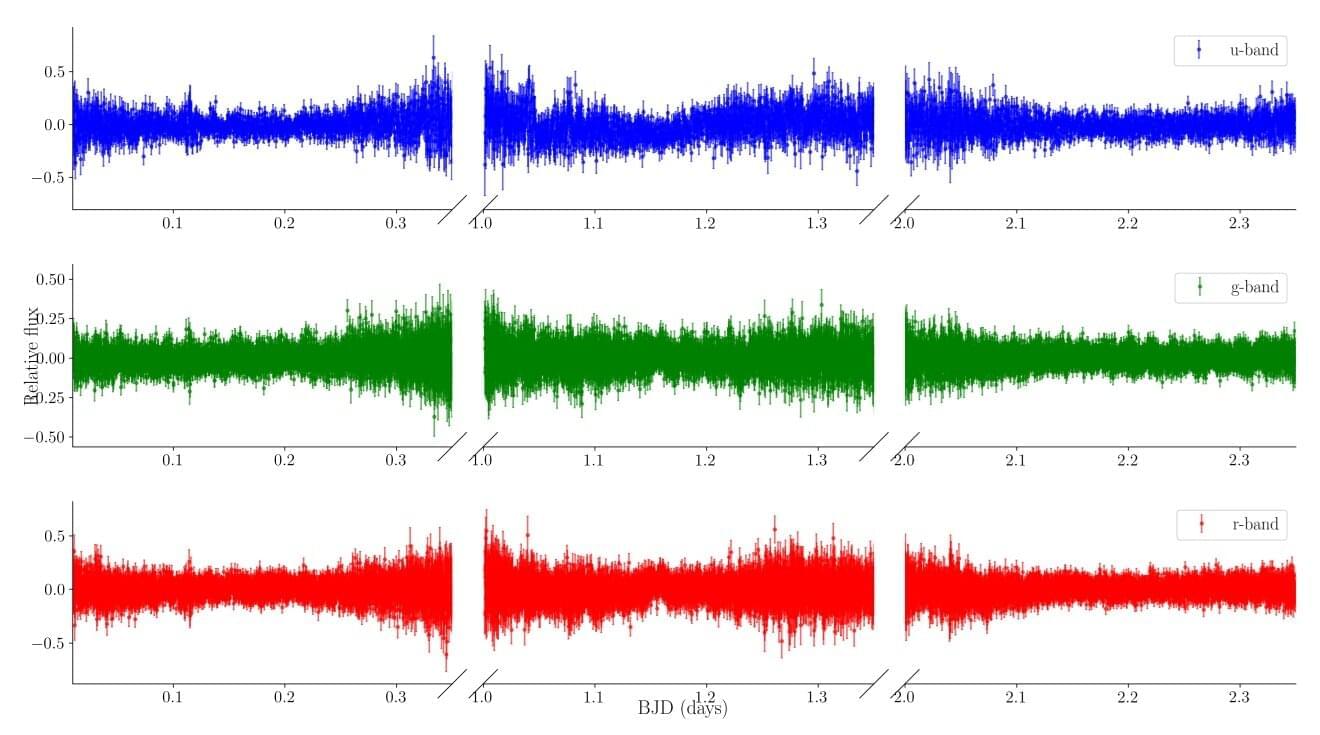
Based on time-series photometry from three different telescopes, an international team of astronomers has performed a detailed asteroseismology study of WD J0049−2525—the most massive pulsating white dwarf. The study, published May 22 on the arXiv pre-print server, resulted in the detection of new pulsation modes of this white dwarf.
White dwarfs (WDs) are stellar cores left behind after a star has exhausted its nuclear fuel and represent the final evolutionary stage for the vast majority of stars. Observations show that most WDs have primary spectral classification DA as they exhibit hydrogen-dominated atmospheres. However, a small fraction of WDs showcases traces of heavier elements.
In pulsating WDs, luminosity varies due to non-radial gravity wave pulsations within these objects. One subtype of pulsating WDs is known as DAVs, or ZZ Ceti stars, which have only hydrogen absorption lines in their spectra.

IN A NUTSHELL 🔬 Rice University researchers discovered copper boride, a novel two-dimensional material with transformative potential. 🧪 The study highlights copper boride’s strong covalent bonding and distinct electronic properties, setting it apart from other 2D materials. 🔋 This breakthrough could significantly impact electrochemical energy storage and applications in quantum information technology. 🌟 The discovery