To Food and Beyond: How Synthetic Biology will Revolutionize the Space Food Sector in the Future
by Lifeboat Foundation Advisory Board member Guido Putignano.
86,400 not only are the seconds in a day, but also they are the amount of dollars space agencies invest in feeding one astronaut every week. It’s as if space agencies would buy a house every week that accidentally disappears when you buy it. Time to grow food in space instead!
In this report, we will talk about
Plant-Based Meat
Cultivated Meat
Stem Cells
Cultivated Meat Production
Future Possibilities
Introduction
Imagining in 20 years from now, space will become something common, expecting to reduce that cost would make anything easier.
Indeed, many organizations like ESA launched different calls to ask for proposals and organizations like Aleph Farms already made that possibility a reality.

Indeed, Aleph Farms is one of the most influential organizations in Cultivated Meat. They launched Aleph Zero, focusing on “advancing food security by producing fresh quality meat anywhere, independent of climate change and availability of local natural resources.”
Whenever we have to think about food, one of the most important points is meat.
Currently, we have 2 main possibilities for meat production.
Plant-based Meat
Cultivated Meat
Plant-based Meat
Plant-based refers to products made from plants that are alternatives to animal-based products. This includes plant-based meat, seafood, eggs, and dairy.
The concept of plant-based meat has always been present.
The prevalence and variety of plant-based meat has steadily increased for centuries. However, many early plant-based meat products were designed with vegetarian consumers in mind, without thinking about other potential needs. For this reason, they did not try to replicate exactly, or biomimic, conventional meat.
If animal meat is primarily muscle tissue, and plants don’t have muscles. How can we make Plant-based Meat possible?
Even if plants don’t have muscles, they contain protein, fat, vitamins, minerals, and water. Indeed, animal meat is made up of protein, fat, vitamins, minerals, and water at its simplest level. Plant-based meat takes advantage of this biochemical similarity between plants and animals.
We can look for an analogue or replacement in the plant kingdom for every protein, lipid, or functional compound in the dozen or two animal species we typically eat. If a replacement doesn’t exist in nature, we can make it through mechanical, chemical, or biological treatment of a plant ingredient.
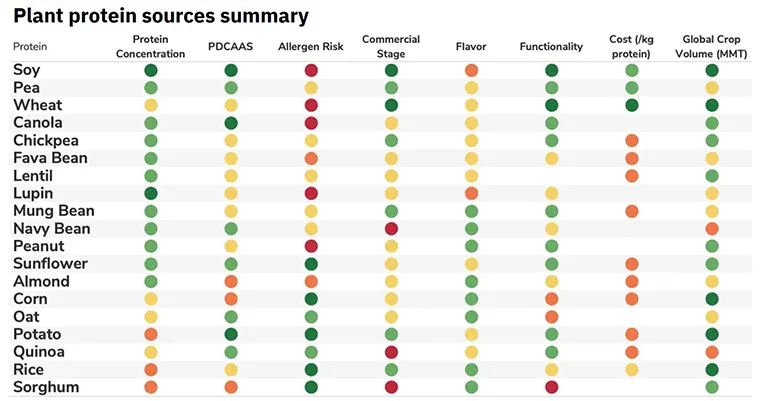
The distinct texture of animal meat isn’t magic; it is all related to the unique spatial arrangement of proteins in muscle tissue. The spatial arrangement of proteins in these whole-muscle types of products is integral to the texture. Thus, not surprisingly, there are more technical challenges to overcome to mimic whole cuts of animal meat with plant ingredients.
The general method used to produce plant-based meat involves three primary steps. First, we grow crops as a source of raw materials. Second, we process these crops to get rid of the parts of the plants we don’t want. At this stage, we end up with the proteins, fats, and fiber ingredients that will become our plant-based meat product. Finally, we put together the desired mixture of ingredients. This ingredient mixture then goes through a manufacturing process to create the muscle-like texture needed for meat.

Even if it can be extremely useful in the short term and on Earth, imagining the need to produce meat in space would be hard to consider.
For this reason, we have to explore another possibility.
Cultivated Meat
Cultured meat aims to replicate conventionally produced meat through stem cells and tissue culture.
Whenever you have to call it, there are different versions. The first one was in-vitro meat, as the cells and tissue are cultured in vitro. In any case, people tend to use the terms cultivated meat, cultured meat, cell-based meat or clean meat to express the same idea
In 2013, professor Mark Post at Maastricht University pioneered a proof-of-concept for cultured meat by creating the first hamburger patty grown directly from cells.
Before that year, in 2001, NASA began conducting experiments on cultured meat, with the intent of allowing far-travelling astronauts to grow meat without sacrificing storage. In partnership with Morris Benjaminson of Turro College, they cultivated pieces of goldfish and, later, turkey.
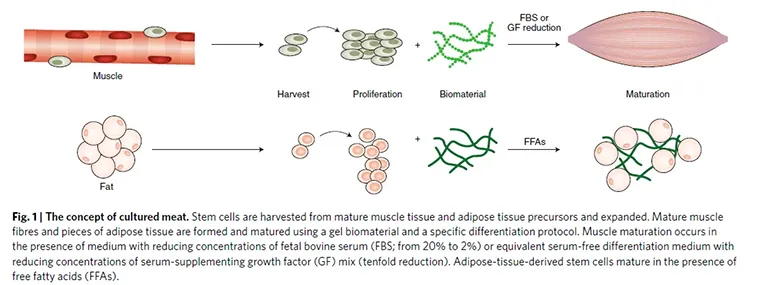
The general way is with scientists that isolate Stem cells from a living animal, expanding them in vitro to produce more of them. Subsequently, cells can differentiate into muscle, fat cells or other types depending on the isolated stem cell type.
Tissue-engineering techniques typically involving a biomaterial scaffold for temporary or permanent support and a way to make cells grow in a three-dimensional environment.
All those activities lead to the assembly of a tissue that is anticipated to resemble meat in its sensory and nutritional qualities in the best possible way.
Before going deeper into all the processes of Cultivated Meat, we need to focus on Stem Cells more.
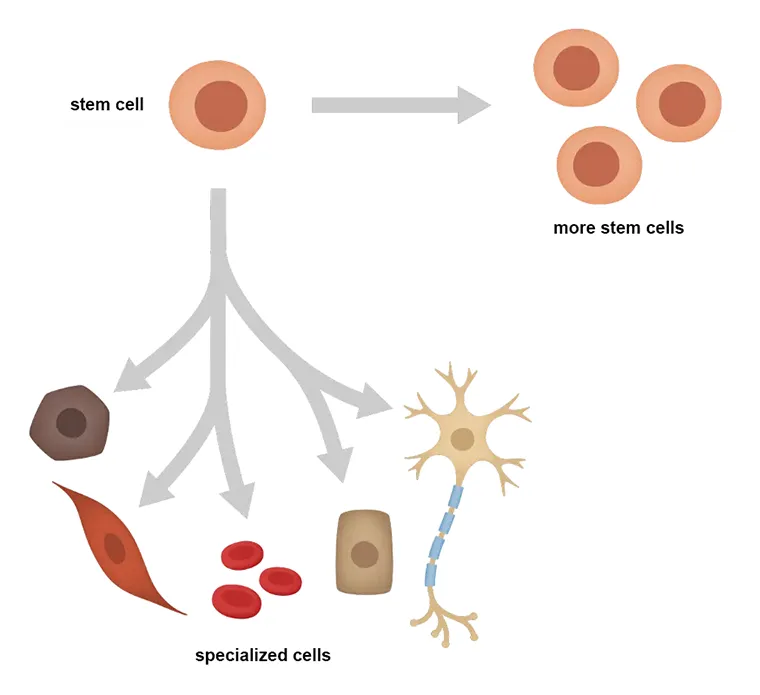
What’s a stem cell?
A stem cell is an unspecialized cell that must be able to be self-renewing and be multipotent. Self-renewing means that the cell must replicate itself into more cells of the same unspecialized cell type. In other words, stem cells can make copies of themselves over and over again.
This term is different from Multipotent cells. Multipotent cells are unspecialized cells and must be able to differentiate into many specialized cell types. These cell types compose the tissues and organs of an individual.
Even if the DNA is always the same, any cell is different for structure or function. That depends on the tissue or the organs.
An old saying goes: “We aren’t all equal”. In a certain way, it is the same with stem cells. There are a variety of different stem cells used in research. Stem cells differ through various times during human development.
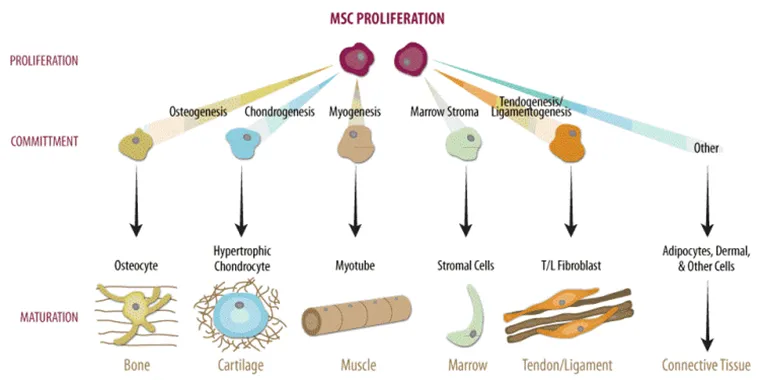
We have 3 types of stem cells:
Embryonic stem cells
Cord Blood stem cells
Adult Stem Cells
Embryonic stem cells are a product of In vitro fertilization. They are made in a petri dish after eggs fertilization, and then they develop into blastocysts.
Cord Blood stem cells are the cells that come from umbilical cords just after a baby is born.
Adult stem cells are found in an adult's body, and they are present in all of us. Scientists have found adult stem cells in the brain, in bone marrows, in the blood, in blood vessels, in skeletal muscles, in the skin and the liver.
What is the difference between these cells?
To answer this question, we should consider what Potency is.
The potency of cells refers to a cell’s ability to make different cell types and germ layers. It comes from the Latin word potent, which means having power. The higher the potency, the higher the number of cell types a cell can differentiate into. Embryonic stem cells are pluripotent because they can become any cell of the body.
Quoting the example made by Dr. Zehra Dincer, if you’re a pluripotent stem cell, you are on the top of a mountain, while you are skiing, you can take many different weights to go down, assuming each path makes another cell type. As pluripotent stem cells, you can differentiate into all the cell types of the body. If you’re a multipotent cell, you’re starting lower down than the pluripotent cell on the potency mountain, such as adult stem cells. You can still pick many ways to go down. However, you can’t go up skiing, so you can only differentiate into a handful of cell types.
Even if there are these 3 types of stem cells, Shinya Yamanaka discovered a fourth important one in Japan. As a child in Osaka, Yamanaka enjoyed taking clocks and radios apart and putting them back together. After beginning his career as a surgeon, he was so frustrated about not being able to heal some of the worst diseases on Earth.
Yamanaka started wondering whether he could reprogram regular adult cells, like skin cells, to become stem cell-like cells. This concept can turn cells pluripotent again. After years of painstaking research, in 2006, Yamanaka and his team discovered that proteins encoded by just four “master genes” could turn any adult cell back into a stem cell. He called these cells Induced Pluripotent Stem Cells or IPSCs. Suppose we want to use the mountain analogy, we can go on the top of the hill again.
Watch the full Shinya Yamanaka video!
Cultivated Meat Production
Going back to our Cultivated Meat, the main idea is to take stems cells with Yamanaka’s factors having IPSCs.
Cultured Meat could be an important step for humankind. We need to recognize how it has been done.
The process of Meat Production relies on:
Cell Selection
Cell Culture Medium
Cell Selection
The biomanufacturing process begins with one or more starting cell populations. The starting cell population may be homogeneous or exhibit heterogeneity. Skeletal muscle cells and adipocytes are the minimal necessary components of cultured meat.
The best cell population should have the capacity for self-renewal and differentiation in an environment where other animal components, such as serum, are minimized or eliminated.
To have differentiation of stem cells to skeletal muscle, scientists follow different protocols.
One approach focuses on culture regimens of growth factors and small-molecule inhibitors to direct cells from the pluripotent state toward the myogenic lineage.
Another possibility employs conditional activation of ectopically expressed transcription factors to program cells to a myogenic lineage from a progenitor state.
In any case, we always have to consider the place where those approaches will be used. Indeed, as seen in Europe, for example, there are regulatory concerns around the combination of genetic modification and cultured meat.
When we consider the cell culture to grow, we need to consider different parameters such as Location, Age, Breed and Sex.
Cell Culture Medium
To extract the cell line, instead, we can underline different procedures such as media composition will define the final characteristics of the cultured meat product.
Metabolic engineering will increasingly rely on constraint-based modelling and flux balance analyses that have been widely applied to predict and quantify the metabolic state of cells.
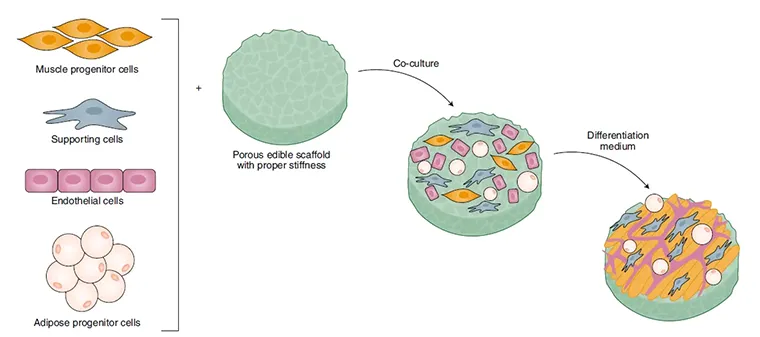
Scientists underline how materials form their structure, supporting cell seeding and 3D tissue formation when discussing scaffolding.
Important for scaffolds to have pores so that the nutrients from the media can reach the cells and allow them to proliferate
In general, scaffolding can be related to different functions:
Biocompatibility where means cells can adhere, migrate, proliferate, etc. the same way they would inside an animal
Biodegradability, essentially this needs to be non-toxic and not create toxic by-products
Mechanical properties: consistency with the important site, structural integrity, etc.
Architecture, it needs to have pores to allow cell infiltration and nutrient diffusion.
Manufacturing Technology, cost-effective, storage, scalability, sustainable, resources efficient

For scaffolds to be useful for cell seeding, they need to follow these guidelines:
Acellular Scaffolds: to use cell-free scaffolds that mimic the extracellular matrix
Biocompatibility: cells must be able to adhere to the surface
Biodegradability: Having products to be able to decompose naturally, as real meat products do too.
Edibility: how can it be manipulated
Mechanical Properties: Needs to have the strength of musculature. Also need to have the porosity to allow media to perfuse to the seeded cells.
Sustainability: How to create more sustainable meat products.
Scalability, cost-effective
Future Possibilities
All that considered, access to fresh food is the main obstacle to long-term space exploration and human colonization: limiting the possibilities and impacting the astronaut’s mental and physiological health. It limits how far away from Earth we can travel and how long we can stay in space since there is a limit to how much food we can stock or initially carry to space. If we are to travel to Mars or the Moon, we need food production systems that are bio-regenerative, and that can locally feed the communities with quality nutrition.

Since 2019, when Aleph Farms made their micro meat tissue be cultivated in space directly from natural cow cells, other space experiments focused on further understanding the effects of microgravity and scarcity of available environmental resources on the efficiency of meat cultivation are taking place.
Maybe, someone of us will be part of that change, too.
To sum up, we have considered the food problem in space underling two possible methods. Cultivated Meat is the most sustainable for different reasons, and we explained its production. Quoting Winston Churchill,
