Page 6761
Mar 28, 2020
Indian scientists imaged novel Coronavirus under a high-powered microscope
Posted by Roderick Reilly in category: biotech/medical
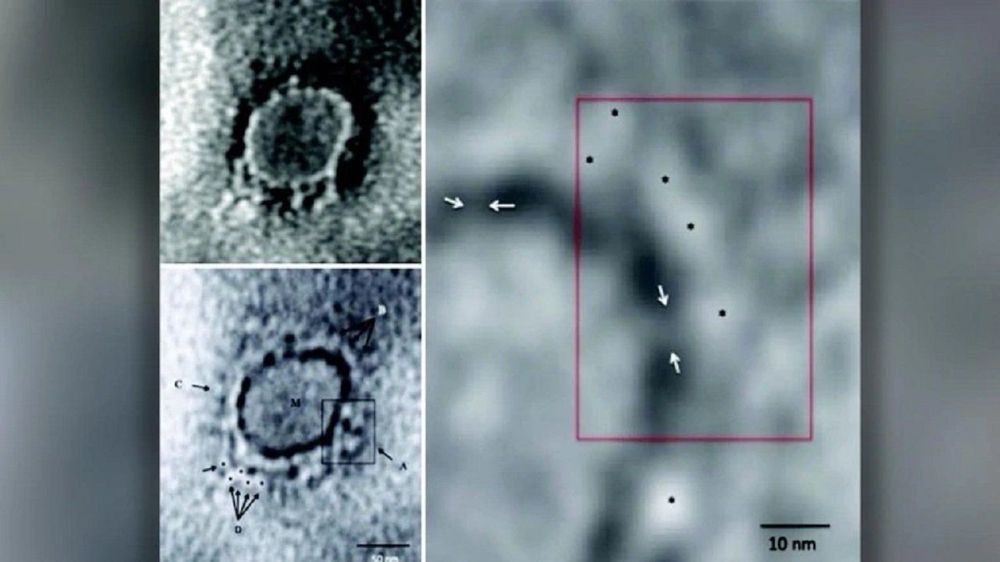
Using transmission electron microscopy (TEM), scientists from the Indian Council of Medical Research (ICMR), managed to image the novel Coronavirus. The image was taken from the throat swab sample of the first laboratory-confirmed novel Coronavirus patient in India.
Scientists tested a total of seven negative-stained virus particles having features of Coronavirus-like particles from the sample.
The novel Coronavirus, which originated in China late last year, has caused a pandemic across the world.
Mar 28, 2020
Video shows phones at a Florida beach during spring break, and where they all traveled during the coronavirus outbreak
Posted by Brent Ellman in categories: biotech/medical, mobile phones
Mar 28, 2020
Pot smokers can maybe breathe a little easier
Posted by Brent Ellman in category: biotech/medical
Moderate consumption of marijuana doesn’t adversely affect lung function, according to a study.
A study published in 2012 in The Journal of the American Medical Association (JAMA) had some good news for people who smoke marijuana: smoking at a rate of one joint a day for as long as seven years doesn’t seem to affect lung function adversely.
Mar 28, 2020
U.S. Approves Abbott Labs Five-Minute ‘Rapid’ Coronavirus Test
Posted by Montie Adkins in category: biotech/medical
Abbott Laboratories won U.S. Food and Drug Administration approval for its molecular test for the Coronavirus strain COVID-19, which the company says can deliver “positive results in as little as five minutes and negative results in 13 minutes.”
The FDA’s “emergency use authorization” awarded to Abbott’s ID NOW COVID-19 test is the latest in a growing number of agency approvals for more rapid molecular “point-of-care” diagnostic tests that can be used in temporary screening locations, doctor’s office labs and nursing homes to detect the Coronavirus strain COVID-19 within a half hour.
“With rapid testing on ID NOW, healthcare providers can perform molecular point-of-care testing outside the traditional four walls of a hospital in outbreak hotspots,” Abbott president and chief operating officer Robert Ford said.
Mar 28, 2020
More than 60,000 people are missing amid Mexico’s drug war, officials say
Posted by Quinn Sena in category: biotech/medical
Mexican authorities admit the number is far higher than previously estimated as murders continue to rise.
Mar 28, 2020
You can now livestream the Northern Lights from your living room
Posted by Genevieve Klien in category: entertainment
While many of us are still trying to figure out how to break up the monotony of self-isolation without spending countless hours in front of the television, perhaps some entertainment courtesy of Mother Nature herself might do the trick?
Aurora borealis (or Northern Lights) is one of nature’s most incredible phenomenons – and now you can livestream it directly into your living room.
Explore.org and Polar Bears International use footage from a camera located in Churchill, Manitoba, Canada, which is situated underneath the aurora oval – thought to be one of the best places on earth to view the aurora borealis.
Mar 28, 2020
A new FDA-authorized COVID-19 test doesn’t need a lab and can produce results in just 5 minutes
Posted by Genevieve Klien in category: biotech/medical
There’s a new COVID-19 test from healthcare technology maker Abbott that looks to be the fastest yet in terms of producing results, and that can do so on the spot right at point-of-care, without requiring a round trip to a lab. This test for the novel coronavirus causing the current global pandemic has received emergency clearance for use by the U.S. Food and Drug Administration, and will begin production next week, with output of 50,000 per day possible starting next week.
The new Abbott ID NOW COVID-19 test uses the Abbott ID NOW diagnostics platform, which is essentially a lab-in-a-box that is roughly the size of a small kitchen appliance. It’s size, and the fact that it can produce either a positive result in just five minutes, or a negative one in under 15, mean that it could be a very useful means to extend coronavirus testing beyond its current availability to more places including clinics and doctor’s offices, and cut down on wait times both in terms of getting tested and receiving a diagnosis.
Unlike the rapid tests that have been used in other countries, and that received a new type of authorization under an FDA guideline that doesn’t confirm the accuracy fo the results, this rapid testing solution uses the molecular testing method, which works with saliva and mucus samples swabbed from a patient. This means that it works by identifying a portion of the virus’ DNA in a patient, which means it’s much better at detecting the actual presence of the virus during infection, whereas other tests that search the blood for antibodies that are used in point-of-care settings can only detect antibodies, which might be present in recovered patients who don’t actively have the virus.
Mar 28, 2020
Astrophysicist Brian May (of Queen!) teaches you how to play the Bohemian Rhapsody solo while in self-isolation
Posted by Genevieve Klien in category: futurism
Brian May is providing tutorials and a gear walkthrough for those practicing guitar in self-isolation.
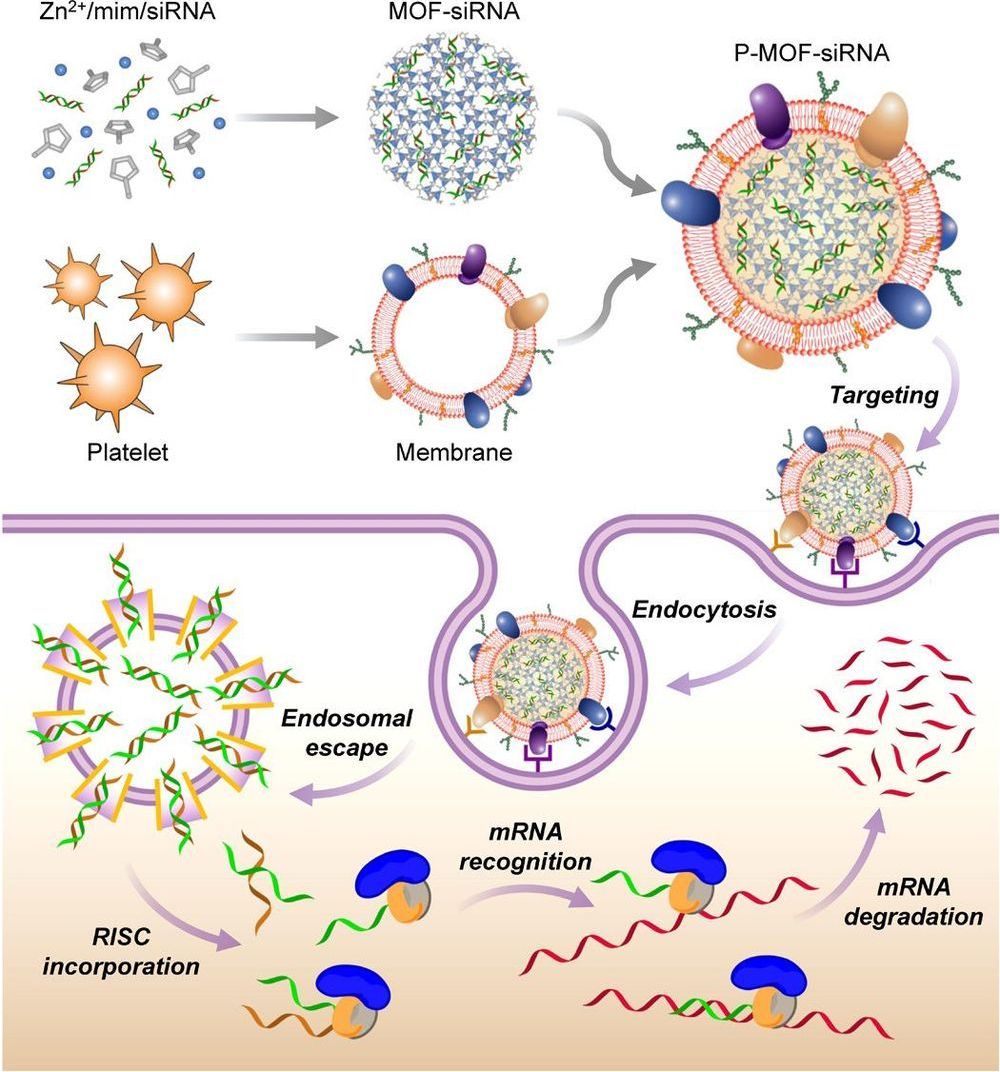
Small interfering RNA (siRNA) is a powerful tool for gene silencing that has been used for a wide range of biomedical applications, but there are many challenges facing its therapeutic use in vivo. Here, we report on a platelet cell membrane–coated metal-organic framework (MOF) nanodelivery platform for the targeted delivery of siRNA in vivo. The MOF core is capable of high loading yields, and its pH sensitivity enables endosomal disruption upon cellular uptake. The cell membrane coating provides a natural means of biointerfacing with disease substrates. It is shown that high silencing efficiency can be achieved in vitro against multiple target genes. Using a murine xenograft model, significant antitumor targeting and therapeutic efficacy are observed. Overall, the biomimetic nanodelivery system presented here provides an effective means of achieving gene silencing in vivo and could be used to expand the applicability of siRNA across a range of disease-relevant applications.
RNA interference (RNAi) is a naturally occurring mechanism for gene down-regulation that, since its first discovery in the late 1990s, has been widely leveraged as a tool for biological studies. Through a robust process mediated by the RNA-induced silencing complex present within the cytosol, target genes can be posttranscriptionally silenced via degradation of the corresponding mRNA. Small interfering RNAs (siRNAs) are short and well-defined double-stranded RNA molecules that can be synthetically manufactured to take advantage of the RNAi pathway. Over time, siRNAs have become an indispensable tool for validating gene function. They have also been widely explored as therapeutics for human disease , and an siRNA-based treatment for transthyretin-mediated amyloidosis was recently approved by the U.S. Food and Drug Administration.